Coronavirus Updates
Is Hydroxychloroquine a cure for COVID-19?
3 min read
By Apollo 24/7, Published on - 21 May 2020, Updated on - 18 October 2022
Share this article
2
2 likes
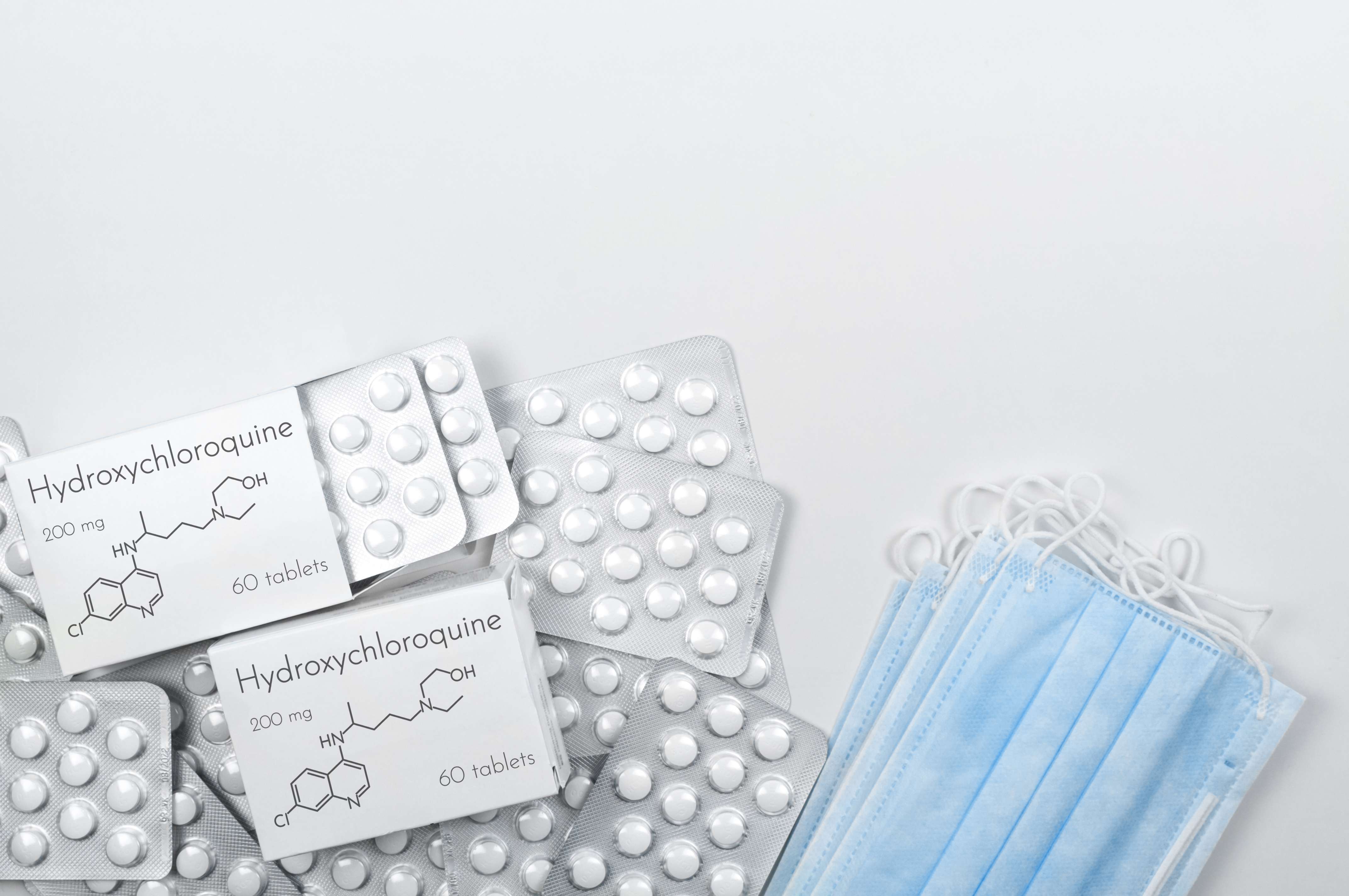
Currently, there is no treatment available for COVID-19 which has affected lakhs of people globally. However, medical experts around the world are trying different treatment options to ease the symptoms in the patients infected with Coronavirus. One treatment option that is presently being explored is the use of Hydroxychloroquine (HCQ).
What is Hydroxychloroquine?
Chloroquine is a drug traditionally used in the treatment and prevention of Malaria. Hydroxychloroquine is a derivative of Chloroquine and is also advised in the treatment of some medical conditions like Rheumatoid arthritis and Systemic lupus erythematosus (SLE). Rheumatoid arthritis is a chronic disease that causes inflammation of joints. SLE is a chronic medical disease that causes inflammation in connective tissues such as cartilage and lining of blood vessels, which provide strength and flexibility to body structures.
Hydroxychloroquine has been studied previously as a potential medicine for viral infections. While its mechanism to act on the infection is not yet proven, it is known to block the entry of the virus into cells of the human body.
A recent study done on the effect of HCQ in severe COVID–19 patients revealed that it did not greatly lower or increase the risk of death in patients. More trials are needed to study this drug further. With multiple drug trials showing similar effects, the United States Food and Drug Administration (FDA) has suggested that HCQ may be given to hospitalized COVID-19 patients with evidence of Pneumonia.
What are the Indian guidelines for the usage of Hydroxychloroquine?
The Indian Council of Medical Research (ICMR) recommends Hydroxychloroquine as prevention against Coronavirus owing to its effectiveness against the virus in laboratory conditions and living organisms. ICMR has recommended HCQ to be given to patients (especially to high-risk individuals) by qualified medical practitioners after carefully considering the risk and benefits.
Which type of individuals can be given HCQ?
- Healthcare workers who do not show any symptoms but are involved in taking care of suspected or confirmed COVID–19 patients
- Contacts of people who have tested positive for Coronavirus but do not show any symptoms.
Hydroxychloroquine is advised to be given only on the prescription of a doctor and recipients need to be monitored for negative reactions. HCQ is known to cause unnatural side effects and sometimes lead to heart rate defects and ultimately death. Hence, it is advised to be used only under the proper supervision of a doctor. It is also never a good idea to self-medicate under any circumstances.
What is the meaning of the Solidarity trial?
Solidarity trial is an international clinical trial launched by the World Health Organization (WHO). In this trial, four treatment options are compared against standard patient care for its effectiveness against Coronavirus. The four treatment options are Remdesivir, Lopinavir/ Ritonavir, Interferon beta-1a, and Chloroquine/ Hydroxychloroquine.
By enrolling patients in multiple countries, this trial aims to rapidly discover medicines or drugs that can either slow the progression of the disease or improve the survival chance of COVID-19 patients. As of April 21, 2020, over 100 countries are working together in this trial to find effective therapeutics for COVID-19.
What is the current situation with the use of Hydroxychloroquine?
Borba and colleagues performed a clinical trial in the Brazilian population to assess the safety of Chloroquine in doses that were thought to have antiviral effects. After the study, it was concluded that Chloroquine is potentially associated with increased deaths in severe COVID–19 patients.
Many other trials are ongoing to study the effectiveness and safety of Hydroxychloroquine. One can only hope that it gives positive results. In the meantime, the trial results in Brazil should be enough to reduce the enthusiasm of the public on the use of Hydroxychloroquine. For the time being, health care workers are discussing potential risk versus uncertain benefits with the patient before initiating treatment.
If you have any questions related to Coronavirus, you can consult our team of expert doctors through online doctor consultation.
Coronavirus Updates
Leave Comment
Recommended for you
Coronavirus Updates
When Should One Get Tested for COVID-19 in India?
You should get yourself tested for COVID-19 if you show any symptoms after undertaking international travel in the last 14 days, had any contact with people who tested positive for the virus, are a patient of SARS, or you are a healthcare worker.

Coronavirus Updates
What Should You Do If You are Exposed to a COVID-19 Patient?
If you’ve met someone recently who has later tested positive for COVID-19, it is natural to be worried about having contracted the virus yourself.

Coronavirus Updates
Oxygen Concentrators: Everything You Need to Know
An oxygen concentrator is a device that provides supplemental oxygen to a patient with breathing issues. It works by filtering and concentrating oxygen from ambient air.
Subscribe
Sign up for our free Health Library Daily Newsletter
Get doctor-approved health tips, news, and more.
Visual Stories
Explained: The Highly Transmissible SARS-CoV-2 Variants
Tap to continue exploring
Recommended for you

Coronavirus Updates
When Should One Get Tested for COVID-19 in India?
You should get yourself tested for COVID-19 if you show any symptoms after undertaking international travel in the last 14 days, had any contact with people who tested positive for the virus, are a patient of SARS, or you are a healthcare worker.

Coronavirus Updates
What Should You Do If You are Exposed to a COVID-19 Patient?
If you’ve met someone recently who has later tested positive for COVID-19, it is natural to be worried about having contracted the virus yourself.

Coronavirus Updates
Oxygen Concentrators: Everything You Need to Know
An oxygen concentrator is a device that provides supplemental oxygen to a patient with breathing issues. It works by filtering and concentrating oxygen from ambient air.